ICSE CLASS 11 CHEMISTRY SYLLABUS
Question Paper Design:
Since Class 11th Chemistry is divided into theory and a practical section. Therefore, there are held two separate exams in chemistry i.e., ‘Theory Exam’ and ‘Practical Exam’.
Scheme For Theory Examination [70 Marks]:
The theory exam is held for total 70 marks with a maximum time limit of 3 hours given to attempt it. The question paper of the theory exam consists of four types of questions, which is listed below.
- Question 1 is of twenty marks having four sub parts all of which are compulsory.
- Question numbers 2 to 8 carry 2 marks with two questions having internal choice.
- Question numbers 9 to 15 carry 3 marks with two questions having internal choice.
- Question numbers 16 to 18 carry 5 marks each. Each question has an internal choice.
| S. No. | Domains | Total Marks | |
| 1 | Remembering and Understanding: Exhibit memory of previously learned material by recalling facts, terms, basic concepts and answers. Demonstrate understanding of facts and ideas by organizing, comparing, translating, interpreting, giving descriptions and stating main ideas. | 28 | 40 |
| 2 | Applying: Solve problems to new situations by applying acquired knowledge, facts, techniques and rules in a different way. | 21 | 30 |
| 3 | Analysing, Evaluating and Creating: Examine and break information into parts by identifying motives or causes. Make inferences and find evidence to support generalizations. Present and defend opinions by making judgments about information, validity of ideas or quality of work based on a set of criteria. Compile information together in a different way by combining elements in a new pattern or proposing alternative solutions. | 21 | 30 |
Practical Exam [15Marks]:
Micro-chemical methods are available for several of the practical experiments. Wherever possible techniques should be used.
Project Work [10 Marks]:
The candidate is to creatively execute one project/assignment on a selected topic of Chemistry. Teachers may assign or students may choose any one project of their choice.
Suggested Evaluation criteria for Project Work:
- Introduction / purpose
- Contents
- Analysis/ material aid (graph, data, structure, pie charts, histograms, diagrams, etc)
- Presentation
- Bibliography
Practical File [05 Marks]:
Teachers are required to assess students based on the Chemistry Practical file maintained by them during the academic year.
Important Links to Access: Other Links to Access:
ISC Class 11 Chemistry Sample Papers ISC Class 11 Physics Syllabus
ISC Class 11 Chemistry Competitive Questions ISC Class 11 Maths Syllabus
ISC Class 11 Chemistry Notes ISC 12th Topic Notes
ISC Class 12 Chemistry Syllabus Other Board Syllabus
Described Syllabus For Theory Exam:
| S. No. | Unit | Total Weightage |
| 1 | Some Basic Concepts of Chemistry | Physical Chemistry 32 Marks |
| 2 | Structure of Atom | |
| 3 | Classification of Elements and Periodicity in Properties | |
| 4 | Chemical Bonding and Molecular Structure | |
| 5 | States of Matter: Gases and Liquids | |
| 6 | Chemical Thermodynamics | |
| 7 | Equilibrium | |
| 8 | Redox Reactions | Inorganic Chemistry 15 Marks |
| 9 | Hydrogen | |
| 10 | s -Block Elements | |
| 11 | Some p -Block Elements | |
| 12 | Organic Chemistry: Some basic Principles and Techniques | Organic Chemistry 23 Marks |
| 13 | Hydrocarbons | |
| 14 | Environmental Chemistry | |
| Total | 70 Marks | |
1. Some Basic Concepts of Chemistry
General introduction: Importance and scope of Chemistry.
Study of matter. Understanding laws of chemical combination. Dalton’s atomic theory: concept of elements, atoms and molecules.
2. Structure of Atom
Bohr’s atomic model and its limitations (deBroglie’s equation, Heisenberg’s uncertainty principle), concept of shells, subshells, orbitals. Quantum numbers, shapes of s, p and d orbitals. Rules for filling electrons in orbitals -aufbau principle, Pauli’s exclusion principle and Hund’s rule of maximum multiplicity. Electronic configuration of atoms, stability of half- filled and completely filled orbitals.
3. Classification of Elements and Periodicity in Properties
Significance of classification; Modern Periodic Law and the present form of periodic table leading to periodic trends in properties of elements – atomic radii, ionic radii, valency, ionisation enthalpy, electron gain enthalpy, electronegativity. Nomenclature of elements with atomic number greater than 100.
4. Chemical Bonding and Molecular structure
Valence electrons, ionic bond character, covalent bond of ionic bond, covalent bond, bond parameters, lewis structure, polar character of covalent bond, VSEPR theory, geometry of covalent molecule s, valence bond theory, concept of hybridisation involving s, p and d orbitals and shapes of some simple molecules. Coordinate bond. Molecular orbital theory of homonuclear diatomic molecules (qualitative idea only). Resonance and hydrogen bond.
5. States of Matter: Gases and Liquids
States of matter and their characteristic properties to establish the concept of the molecule. Boyle’s law, Charles law, Gay Lussac’s law, Avogadro’s law, Avogadro’s number, ideal behaviour of gases and derivation of ideal gas equation. Kinetic Theory of gases. Deviation from ideal behaviour, van der Waal’s equation, liquefaction of gases, critical temperature.
6. Chemical Thermodynamics
(i) First Law of Thermodynamics and its significance, work, heat, internal energy, enthalpy (ΔU or ΔE and ΔH), heat capacity and specific heat. Hess’s law of constant heat summation, enthalpy of bond dissociation, combustion, formation, atomisation, sublimation, phase transition, ionisation, solution and dilution.
(ii) Second Law of Thermodynamics and its significance, spontaneity of a chemical change; Entropy, Free Energy. Inadequacy of First Law and need for Second Law; Ideas about reversible (recapitulation), spontaneous and non-spontaneous processes
(iii) Third Law of Thermodynamics – statement only.
Self-explanatory
7. Equilibrium
(i) Chemical Equilibrium
Introduction of physical and chemical equilibrium and its characteristics Dynamic nature of equilibrium, law of mass action, equilibrium constant and factors affecting equilibrium. Le Chatelier’s principle and its applications.
(ii) Ionic equilibrium
Introduction, electrolyte (strong and weak), non-electrolyte, ionisation, degree of ionisation of polybasic acids, acid strength, concept of pH, pH indicators, buffer solution, common ion effect (with illustrative examples). Henderson equation, hydrolysis of salts, solubility and solubility product.
8. Redox Reactions
Concept of oxidation and reduction, redox reactions, oxidation number, change in oxidation number, balancing redox reactions (in terms of loss and gain of electrons). Applications of redox in various types of chemical reactions
9. Hydrogen
Hydrogen and its compounds: hydrides, water, heavy water, hydrogen peroxide.
10. s-Block Elements (Alkali and Alkaline Earth Metals)
(i) Group 1 and 2 elements
General characterises of Group 1 and 2 should include the following:
Occurrence; physical state; electronic configuration; atomic and ionic radii; common oxidation state; electropositive /electronegative character; ionisation enthalpy; reducing/oxidising nature.
(ii) Preparation and properties of some important compounds
Sodium chloride, sodium hydroxide, Sodium carbonate, sodium bicarbonate, sodium thiosulphate; biological importance of sodium and potassium.
Magnesium chloride hexahydrate, calcium oxide, calcium hydroxide, calcium carbonate, plaster of paris and cement. Industrial uses of the above, biological importance of magnesium and calcium.
11. Some p -Block Elements
(i). Group 13 Elements
General introduction, electronic configuration, occurrence, variation of properties, oxidation states, trends in chemical reactivity, physical and chemical properties.
Preparation and properties of some important compounds, boric acid, aluminium: Reactions with acids and alkalies. Lewis acid character of boron halides; amphoteric nature of aluminium, alums. Boric acid – preparation and action of heat. Aluminium: Reactions with acids and alkalies. Alums – preparation and uses.
(ii). Group 14 Elements
General characteristics, electronic configuration, occurrence, variation of properties, oxidation states, trends in chemical reactivity.
Preparation and properties of: Silicon carbide – preparation from silica. Uses. Silicones – general method of preparation. Uses.
12. Organic Chemistry – Some Basic Principles and Techniques
General introduction, classification and IUPAC nomenclature of organic compounds and isomerism. Qualitative and quantitative analysis. Electron displacement in a covalent bond: inductive effect, electrometric effect, resonance and hyperconjugation. Homolytic and heterolytic bond fission of a covalent bond: free radicals, carbocations, carbanions, electrophiles and nucleophiles, types of organic reactions.
13. Hydrocarbons
Classification of Hydrocarbons:
1. Aliphatic Hydrocarbons
(i) Alkanes – Nomenclature, isomerism, physical properties, chemical properties including free radical mechanism of
halogenation, combustion and pyrolysis.
(ii) Alkenes – Nomenclature, structure of double bond (ethene), isomerism; methods of preparation; physical properties,
chemical properties; addition of hydrogen, halogen, water, hydrogen halides (Markownikoff’s addition and peroxide
effect), ozonolysis, oxidation
(iii) Alkynes – Nomenclature, structure of triple bond (ethyne), methods of preparation; physical properties, chemical properties: acidic character of alkynes, addition reactions-hydrogen, halogens, hydrogen halides and water.
2. Aromatic Hydrocarbons
Introduction, IUPAC nomenclature, benzene: resonance, aromaticity, chemical properties: Nitration, sulphonation,
halogenation, Friedel Crafts alkylation and acylation, directive influence of functional group in monosubstituted benzene.
14. Environmental Chemistry
Types of environmental pollution (air, water and soil pollution); various types of pollutants: smog, acid rain; effects of depletion of ozone layer, greenhouse effect and global warming. Pollution due to industrial wastes, green Chemistry as an alternative tool for reducing pollution; strategies for control of environmental pollution.
Described Syllabus For Practical Exam:
Candidates are required to complete the following experiments:
A. Titration: acid-base titration involving molarity. Titrations involving:
- Sodium carbonate solution/ dil H2SO4 or dil. HCl using methyl orange indicator.
- NaOH or KOH solution/ dil H2SO4 or dil. HCl using methyl orange indicator.
- Calculations involving molarity, concentration in grams L-1/ number of ions, water of crystallisation and percentage purity.
NOTE: Calculation of molarity must be upto 4 decimal places at least, in order to avoid error.
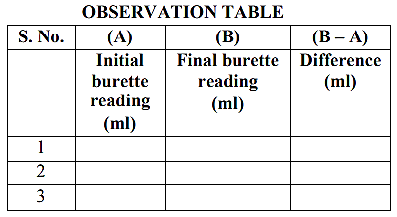
- Concordant reading is to be used for titre value. Concordant reading is two consecutive values which are exactly the same. Average will not be accepted as titre value.
- The table is to be completed in ink only. Pencil is not to be used.
- Overwriting will not be accepted in the tabular column.
Observations:
- Pipette size (should be same for all the candidates at the centre):
- Titre value (concordant).
B. Qualitative analysis: identification of single salt containing one anion and one cation:
- Anions: CO32-, NO2 – , S2-, SO32-, SO42-, NO3 – , CH3COO– , Cl– , Br– , I– , C2O42-, PO43-.
- Cations: NH4+, Pb2+, Cu2+, Al3+, Fe3+, Zn2+, Mn2+ Ni2+, Co2+, Ba2+, Sr2+, Ca2+, Mg2+.
Anions:
- Dilute acid group – CO32-, NO2 – , S2-, SO32-
- Concentrated Acid Group – NO3 – , CH3COO– , Cl– , Br– , I–
- Special Group – SO42-, PO43-, C2O42-.
Cations:
- Group Zero: NH4+
- Group I: Pb2+
- Group II : Cu2+, Pb2+
- Group III: Al3+, Fe3+
- Group IV: Zn2+, Mn2+, Ni2+, Co2+
- Group V: Ba2+, Sr2+, Ca2+
- Group VI: Mg2+
NOTE:
- For wet test of anions, sodium carbonate extract must be used (except for carbonate).
- Chromyl chloride test not to be performed.
(Insoluble salts, such as lead sulphate, barium sulphate, calcium sulphate, strontium sulphate should not be given).
Suggested Books:
- ISC Nootan Chemistry Class 11 by HC Srivastava.
- S Chand ISC Chemistry for Class 11 by RD Madan and BS Bisht.
- KL Chugh Chemistry Class 11 for Physical and Inorganic Chemistry by KL Chugh.
- Modern’s ABC Of Practical Chemistry For Class-11


