Question Paper Design:
Since Class 12th Chemistry is divided into theory and a practical section. Therefore, there are held two separate exams in chemistry i.e., ‘Theory Exam’ and ‘Practical Exam’.
Scheme For Theory Examination [70 Marks]:
There will be no overall choice in the paper. Candidates will be required to answer all questions. Internal choice will be available in two questions of 2 marks each, two questions of 3 marks each and all the three questions of 5 marks each.
| S. No. | Domains | Total Marks | |
| 1 | Remembering and Understanding: Exhibit memory of previously learned material by recalling facts, terms, basic concepts and answers. Demonstrate understanding of facts and ideas by organizing, comparing, translating, interpreting, giving descriptions and stating main ideas. | 28 | 40 |
| 2 | Applying: Solve problems to new situations by applying acquired knowledge, facts, techniques and rules in a different way. | 21 | 30 |
| 3 | Analysing, Evaluating and Creating: Examine and break information into parts by identifying motives or causes. Make inferences and find evidence to support generalizations. Present and defend opinions by making judgments about information, validity of ideas or quality of work based on a set of criteria. Compile information together in a different way by combining elements in a new pattern or proposing alternative solutions. | 21 | 30 |
Practical Exam [15Marks]:
Question 1: Volumetric Analysis
Question 2: Any one or a combination of the following experiments:
- Study of the rate of reaction.
- Identification of the organic compounds and functional groups based on observations.
- Characteristic tests of carbohydrates and proteins.
- Experiments related to pH determination using pH paper or universal indicator.
Question 3: Qualitative Analysis (single salt).
Project Work [10 Marks]:
The project work is to be assessed by a Visiting Examiner appointed locally and approved by the Council.
The candidate is to creatively execute one project/assignment on an aspect of Chemistry. Teachers may assign or students may select a topic of their choice. Following is only a suggestive list of projects.
Suggested Evaluation criteria for Project Work:
- Introduction / purpose
- Contents
- Analysis/ material aid (graph, data, structure, pie charts, histograms, diagrams, etc)
- Presentation
- Bibliography
Practical File [05 Marks]:
The Visiting Examiner is required to assess students on the basis of the Chemistry Practical file maintained by them during the academic year.
Important Links to Access:
ISC Class 12 Chemistry Sample Papers
ISC Class 12 Chemistry Competitive Questions
ISC Class 12 Chemistry Notes
ISC Class 11 Chemistry Syllabus
Other Links to Access:
ISC Class 12 Physics Syllabus
ISC Class 12 Maths Syllabus
ISC 11th Topic Notes
Other Board Syllabus
Described Syllabus For Theory Exam:
| S. No. | Unit | Total Weightage |
| 1 | Solid State | Physical Chemistry
25 Marks |
| 2 | Solutions | |
| 3 | Electrochemistry | |
| 4 | Chemical Kinetics | |
| 5 | Surface Chemistry | |
| 6 | General Principles and Processes of Isolation ofElements | |
| 7 | p -Block Elements | |
| 8 | d -and f -Block Element | Inorganic Chemistry
20 Marks |
| 9 | Coordination Compounds | |
| 10 | Haloalkanes and Haloarenes | |
| 11 | Alcohols, Phenols and Ethers | |
| 12 | Aldehydes, Ketones and CarboxylicAcids | |
| 13 | Organic Compounds containing Nitrogen | |
| 14 | Biomolecules | Organic Chemistry
25 Marks |
| 15 | Polymers | |
| 16 | Chemistry in Everyday Life | |
| Total | 70 Marks | |
1. Solid State
Solids: their classification based on different binding forces such as: ionic, covalent molecular; amorphous and crystalline solids (difference), metals. Type of unit cell in two dimensional and three dimensional lattices, number of atoms per unit cell (all types). Calculation of density of unit cell, packing in solids, packing efficiency, voids, point defects, electrical and magnetic properties.
Band theory of metals. Conductors, semiconductors (n and p type) and insulators.
- Crystalline and amorphous solids.
- Definition of crystal lattice, unit cell; types of unit cell (scc, fcc, bcc); calculation of the number of atoms per unit cell; relationship between radius, edge length and nearest neighbour distance. Calculation of density of unit cell, formula of the compound – numericals based on it; packing in 3D, packing fraction in scc, fcc, bcc with derivation; voids – types, location, formation (derivation of radius of voids).
- Characteristics of crystalline solids; ionic (NaCl), metallic (Cu), atomic (diamond and graphite)
- Point defects: Stoichiometric, nonstoichiometric and impurity defects (F- centres).
- Electrical properties: Conductors, semiconductors (n & p types) and insulators (Band Theory), piezoelectricity and pyroelectricity.
- Magnetic properties: diamagnetic, paramagnetic, ferromagnetic, ferrimagnetic and antiferromagnetic.
2. Solutions
Study of concentration of solutions of solids in liquids, liquid in liquid, solubility of gases in liquids, solid solutions, Colligative properties – Raoult’s law of relative lowering of vapour pressure (1st & 2nd), elevation of boiling point, depression of freezing point, osmotic pressure. Use of colligative properties in determining molecular masses of solutes.
Normality, molality, molarity, mole fraction, ppm, as measures of concentration. Definition of the above with examples. Simple problems based on the above.
- Solubility of gases in liquids – Henry’s Law, simple numericals based on the above.
- Raoult’s Law for volatile solutes and nonvolatile solutes, ideal solution, non-ideal solution. Azeotropic mixtures – definition, types, graphical representation, fractional distillation with examples.
- Colligative properties – definition and examples, and its use in determination of molecular mass.
- Relative lowering of vapour pressure: Definition and mathematical expression of Raoult’s Law. Determination of relative molecular mass by measurement of lowering of vapour pressure
- Depression in freezing point: molal depression constant (cryoscopic constant) – definition and mathematical expression (derivation included).
- Elevation in boiling point method: molal elevation constant (ebullioscopic constant) definition and mathematical expression (derivation included).
- Osmotic pressure: definition and explanation. Natural and chemical semipermeable membranes, reverse osmosis, isotonic, hypotonic and hypertonic solutions. Comparison between diffusion and osmosis. Application of osmotic pressure in the determination of relative molecular mass.
van’t Hoff- Boyle’s Law, van’t Hoff – Charles’ Law, van’t Hoff – Avogadro’s law. - Abnormal molecular mass: Dissociation and Association with suitable examples
- Van’t Hoff factor for the electrolytes which dissociate and the molecules which associate in solution. Modification of the formula of colligative properties based on van’t Hoff factor. Simple problems. Calculation of degree of dissociation and association. Experimental details not required.
3. Electrochemistry
Electrolytic and electrochemical cells. Redox reactions in electrochemical cells. Electromotive Force (emf) of a cell, standard electrode potential, Nernst equation and its application to chemical cells. Relation between Gibbs energy change and emf of a cell.
Conductance in electrolytic solutions, specific, equivalent and molar conductivity, variations of conductivity with concentration, graphs; Kohlrausch’s Law of electrolysis and Faraday’s Laws of electrolysis. Dry cell and lead accumulator, fuel cells, corrosion.
- Electrochemical cells: introduction, redox reactions (principle of oxidation and reduction in a cell).
- Galvanic cells – introduction; representation, principle – oxidation reduction. Mechanism of production of electric current in a galvanic cell.
- Measurement of potential. Single electrode potentials. Standard hydrogen electrode (Eo ) – definition, preparation, application and limitations. Standard electrode potential – Measurement of standard electrode potential of Zn ++ / Zn , Cu ++ / Cu, half cell (using standard hydrogen electrode). Cell notation – representation. Factors affecting electrode potential with explanation – main emphasis on the temperature, concentration and nature of the electrode.
- Electrochemical series. Its explanation on the basis of standard reduction potential. Prediction of the feasibility of a reaction.
- Nernst equation and correlation with the free energy of the reaction with suitable examples. Prediction of spontaneity of a reaction based on the cell emf. Numericals on standard electrode potential of half-cells, cell emf, relationship between free energy and equilibrium constant, standard electrode potential and free energy.
- Comparison of metallic conductance and electrolytic conductance. Relationship between conductance and resistance. Specific resistance and specific conductance. Cell constant: Calculation of cell constant. Meaning of equivalent conductance. Meaning of molar conductance. General relationship between specific conductance, molar conductance and equivalent conductance (units and graphs). Units, numericals. Molar conductance of a weak electrolyte at a given concentration and at infinite dilution. Kohlrausch’s Law – definition, applications and numericals.
- Faraday’s laws of Electrolysis.
Faraday’s First Law of electrolysis. Statement
, mathematical form. Simple problems. Faraday’s Second Law of electrolysis: Statement, mathematical form. Simple problems. Relation between Faraday, Avogadro’s number and charge on an electron. F = NAe should be given (no details of Millikan’s experiment are required). - Batteries: Primary and Secondary Cells: Leclanche cell, mercury cell, Lead storage battery and fuel cell – structure, reactions and uses.
- Corrosion: Concept, mechanism of electrochemical reaction, factors affecting it and its prevention.
4. Chemical Kinetics
Meaning of Chemical Kinetics – slow and fast reactions. Rate of a reaction – average and instantaneous rate (graphical representation). Factors affecting rate of reaction: surface area, nature of reactants, concentration, temperature, catalyst and radiation. Order and molecularity of a reaction, rate law and specific rate constant. Integrated rate equations and half-life (only for zero and first order reactions), concept of collision theory (elementary idea, no mathematical treatment). Concept of threshold and activation energy, Arrhenius equation.
- Meaning of chemical kinetics, Scope and importance of Kinetics of the reaction, slow and fast reactions – explanation in terms of bonds.
- Rate of Reaction: definition, representation of rate of reaction in terms of reactants and products, determination of rate of reactions graphically, instantaneous and average rate of reaction. Factors affecting rate of reaction
- Law of mass Action: statement and meaning of active mass. Explanation with an example – general reactions.
- Effect of concentration of reactants on the rate of a reaction: Qualitative treatment, based on the law of mass Action, statement of rate law, General rate equation – Rate = k(concentration of the reactant)n , where k is rate constant and n is the order of the reaction, relationship between the rate of the reaction with rate constant with respect to various reactants.
- Order of a reaction: meaning, relation between order and stoichiometric coefficients in balanced equations, order as an experimental quantity, rate equation for zero order reaction and its unit, mathematical derivation of rate equation for first order reaction, characteristics of first order reaction – rate constant is independent of the initial concentration, units to be derived, definition of half-life period, derivation of expression of half-life period from first order rate equation.
Problems based on first order rate equation and half-life period. - Molecularity of the reaction: Meaning – physical picture, Relation between order, molecularity and the rate of a reaction, Differences between order and molecularity of a reaction.
- The concept of energy: Exothermic and endothermic reactions, concept of energy barrier, threshold and activation energy, formation of activated complex, effect of catalyst on activation energy and reaction rate.
- Collision Theory: Condition for a chemical change – close contact, particles should collide. Collisions to be effective – optimum energy and proper orientation during collision. Energy barrier built-up when the collision is about to take place, Activated complex formation, difference in energy of the reactant and the product – exothermic and endothermic reactions with proper graphs and labelling.
- Mechanism of the reaction: meaning of elementary reaction, meaning of complex and overall reaction, explanation of the mechanism of the reaction, slowest step of the reaction. Relationship between the rate expression, order of reactants and products at the rate-determining step, units of rate constant – explanation with suitable examples
Effect of temperature on the rate constant of a reaction: Arrhenius equation – K=Ae-Ea/RT, Meaning of the symbols of Arrhenius equation, related graph, evaluation of Ea and A from the graph, meaning of slope of the graph, conversion from exponential to log form of the equation, relationship between the increase in temperature and the number of collisions. Numerical based on Arrhenius equation.
5. Surface Chemistry
Absorption and Adsorption – physisorption and chemisorption, factors affecting adsorption of gases on solids and liquids. Catalysis; homogenous and heterogenous, activity and selectivity.
Colloidal state distinction between true solutions, colloids and suspension; lyophilic, lyophobic multimolecular, macromolecular and associated colloids; properties of colloids; Brownian movement, Tyndall effect, coagulation and electrophoresis. Emulsion – types of emulsions.
- Difference between absorption and adsorption: definition of physisorption and chemisorption and their differences.Factors affecting adsorption of gases on solids.
- Catalysis: definition, types of catalysts – positive and negative, homogeneous and heterogeneous catalyst based on the state of the reactant and the catalyst, Elementary treatment of intermediate compound formation theory with examples; adsorption Theory, effect of catalyst on the rate of reaction – the change in the energy of activation in the activation energy curve. Characteristics of a catalyst; specificity, activity, surface area of a catalyst. Promoter and poison.
Colloidal State: Thomas Graham classified the substances as crystalloid and colloid, classification of substances on the basis of the particle size i.e. true solution, sol and suspension, colloidal system is heterogeneous. lyophilic and lyophobic colloid;, classification of colloidal solutions as micro, macro and associated colloids.
Preparation of lyophilic colloids. Preparation of lyophobic colloids by colloid mill, peptization, Bredig’s arc method, oxidation, reduction, double decomposition and exchange of solvent method, purification of colloids (dialysis, ultrafiltration, and ultracentrifugation).
Properties of colloidal solutions:Brownian movement, Tyndall effect, coagulation, electrophoresis (movement of dispersed phase), Protection of colloids, Gold number and Hardy- Schulze rule. Emulsions, surfactants, micelles (only definition and examples).
Application of colloids and emulsions in daily life.
6. General Principles and Processes of Isolation of Elements
Metals: metallurgy, ores, principles and methods of extraction – concentration, oxidation, reduction, electrolytic refining. Occurrence and principles of extraction of aluminium, copper, zinc, iron and silver.
- Definition of minerals, ores and metallurgy; principle ores of aluminium, iron, copper, zinc and silver.
Methods of concentration of ores: hydraulic washing, magnetic separation, froth floatation method, leaching.
Extraction of metal from concentrated ore – calcination, roasting and thermal reduction.
Metallurgy of aluminium, iron, copper, zinc and silver.
Refining of metals – distillation, liquation, electrolysis, vapour phase refining (nickel), zone refining. - Uses of metals and their alloys.
7. p-Block Elements
Group-15 Elements
Position in the periodic table, occurrence, electronic configuration, oxidation states, trends in physical and chemical properties. Nitrogen: preparation properties and its uses; compounds of nitrogen: oxides of nitrogen. Ammonia and nitric acid – preparation and properties. Phosphorus – allotropic forms, compounds of phosphorus: preparation and properties of phosphine, halides and oxoacids.
- General introduction, electronic configuration, occurrence, oxidation states. Trends in physical properties; chemical properties with hydrogen, oxygen and halogens.
- Nitrogen – Laboratory preparation, decomposition (ammonium dichromate, barium azide). Properties and uses.
- Oxides of nitrogen (N2O, NO, N2O3, N2O4, N2O5) – preparation, structure and uses.
- Ammonia – Preparation and manufacture. Properties: reaction with oxygen, copper oxide, chlorine, hydrochloric acid, formation of complexes. Uses.
- Nitric Acid – Pre
paration and manufacture. Properties: reaction with copper (dilute and concentrated HNO3), carbon and sulphur. Uses. - Allotropes of phosphorus and their structures.
Phosphine – Preparation from phosphorus and properties: reaction with halo acids.
Phosphorus trichloride – Preparation from phosphorous. Uses. Phosphorus pentachloride – preparation from PCl3. Thermal dissociation and hydrolysis. Uses, properties. Oxoacids of phosphorus (structures and preparation only).
Group-16 Elements
Position in the periodic table, occurrence, electronic configuration, oxidation states, trends in physical and chemical properties. Oxygen: methods of preparation, properties and uses, classification of oxides. Ozone – methods of preparation. Sulphur -allotropic forms. Compounds of sulphur: preparation, properties and uses of sulphur dioxide (industrial process of manufacture). Oxoacids of sulphur (structuresonly).
- Electronic configuration, oxidation states, occurrence. Trends in physical properties; chemical properties with hydrogen, oxygen and halogens.
- Oxygen – lab method of preparation, formation of oxides with metals and nonmetals and their common nature.
- Ozone: manufacture by Siemen’s ozoniser, thermal decomposition of ozone, its oxidising nature – reaction with lead sulphide, potassium iodide and mercury, its uses.
- Sulphur dioxide: laboratory and industrial preparation from sulphites and sulphide ores, reaction of sulphur dioxide with NaOH, Cl2, KMnO4 and structure of SO2.
- Oxoacids of sulphur: structures only.
Sulphuric Acid: manufacture by Contact Process (equations, conditions and diagram), properties – acidic nature, mode of dilution, oxidising action, dehydrating nature and uses of sulphuric acid in industry.
Group-17 Elements
Position in the periodic table, occurrence, electronic configuration, oxidation states, trends in physical and chemical properties; Preparation, properties and uses of chlorine and hydrochloric acid. Compound of halogen, oxoacids of halogens (structures only), Interhalogen compounds.
- General introduction, electronic configuration, oxidation states. Trends in physical properties and chemical properties (hydrogen, oxygen, halogens and metals).
- Chlorine – preparation from MnO2 and HCl, from NaCl, MnO2 and conc. H2SO4 (only equations), reactions of chlorine with H2S, NH3, cold, dilute NaOH and hot, concentrated NaOH.
- Hydrochloric acid: Lab preparation, its acidic nature, reaction with ammonia, carbonates and sulphites, formation of aqua regia and its uses.
- Oxoacids of halogens: structures and acidic property.
- Interhalogen compounds – structure, hybridisation and shapes: XX′, XX′3, XX′5, XX′7.
Group-18 Elements
Position in the periodic table, occurrence, electronic configuration, trends in physical and chemical properties, inert nature, uses.
- General introduction, electronic configuration, occurrence, trends in physical; chemical properties, state and low reactivity.
- Formation of xenon compounds with fluorine and oxygen (equations only), hybridisation, shape and structure of compounds.
- Uses of noble gases
8. d and f Block Elements
Position in the periodic table, occurrence, electronic configuration and characteristics of transition metals, general trends in properties of the 3d-series of transition metals – metallic character, ionisation enthalpy, oxidation states, ionic radii, colour of ions, catalytic property, magnetic properties, interstitial compounds, alloy formation, preparation and properties of K2Cr2O7 and KMnO4.
Lanthanoids and actinoids.
- d-Block: 3d, 4d and 5d series Study in terms of metallic character, atomic and ionic radii, ionisation enthalpy, oxidation states, variable valency, formation of coloured compounds, formation of complexes, alloy formation.
- f-Block: 4f and 5f series Electronic configuration, atomic and ionic radii, oxidation states, formation of coloured compounds, formation of complexes, alloy formation. Lanthanide contraction and its consequences. Chemical reactivity – with oxygen, hydrogen, halogen, sulphur, nitrogen, carbon and water. Actinides – oxidation states and comparison with lanthanoids.
- Potassium permanganate: structure, shape, equation of extraction from pyrolusite ore, its oxidising nature in acidic, basic and neutral medium, use in redox titration. Oxidising nature in acidic [FeSO4, (COOH)2.2H2O, KI], basic (KI) and neutral (H2S) mediums to be done.
Potassium dichromate: structure, shape, equation of extraction from chromite ore and its use in titration. Oxidising nature in acidic, basic and neutral medium, use in redox titration.
9. Coordination Compounds
Concept of complexes, definition of ligands, coordination number, oxidation number. IUPAC nomenclature of mononuclear coordination compounds. Isomerism (structural and stereo). Bonding, Werner’s theory, VBT and CFT. Colour, magnetic properties and shapes. Importance of coordination compounds (in qualitative analysis, extraction of metals and biological system).
- Definition of coordination compounds / complex compounds, differences with a double salt, study of ligands – mono-, bi-, tri, tetra-, penta-, hexa- and polydentate, chelating ligands, definition of coordination number, its calculation for a complex coordination sphere, study of oxidation state of an element in a complex, its calculation, IUPAC rules of nomenclature of coordination compounds.
- Isomerism – structural, stereo types and examples.
- Valence bond theory of coordination compounds – examples of formation of inner orbital and outer orbital complexes (high and low spin, octahedral, tetrahedral and square planar), prediction of magnetic character.
- Crystal field theory – crystal field splitting in tetra and octahedral systems. Explanation of colour and magnetic character.
- Stability of coordination compounds (explain stability on the basis of magnitude of K) as mentioned above).
- Importance and uses
10. Haloalkanes and Haloarenes:
Haloalkanes: General formula, nomenclature and classification. Nature of C–X bond, physical and chemical properties, mechanism of substitution reactions, optical rotation.
Haloarenes: Basic idea, nature of C–X bond, substitution reactions (directive influence of halogen in monosubstituted compounds only).
Nature of C-X bond
Naming the halogen derivatives of alkanes by using common system and IUPAC system for mono, di and tri-halo derivatives.
Preparation of haloalkanes from:
- Alkane and halogen
- Alkene and hydrogen halide.
- Alcohols with PX3, PCl5and SOCl2.
- Halide exchange method (Finkelstein and Swarts)
- Silver salt of fatty acids (Hunsdiecker).
Physical properties: State, melting point, boiling point and solubility.
Chemical properties: nucleophilic substitution reactions (SN1, SN2 mechanism in terms of primary, secondary and tertiary halides)
Reaction with: sodium hydroxide, water, sodium iodide, ammonia, primary amine, secondary amine, potassium cyanide, silver cyanide, potassium nitrite, silver nitrite, silver salt of fatty acid and lithium-aluminium hydride.
Elimination reaction (Saytzeff’s rule) / β elimination.
Reaction with metals: sodium and magnesium (Wurtz’s reaction, Grignard’s reagent preparation).
Chloroform and iodoform: preparation and properties.
Preparation of haloarenes by Sandmeyer and Gattermann reaction, by electrophilic substitution.
Physical properties: State, melting point, boiling point and solubility.
Chemical properties:
- Electrophilic substitution (chlorination nitration and sulphonation) with mechanism.
- Nucleophilic substitution (replacement of chlorine with -OH, -NH2) with mechanism.
- Reduction to benzene.
- Wurtz-Fittig reaction.
- Fittig reaction.
- Addition reaction with magnesium (formation of Grignard reagent).
- Structure of DDT.
11. Alcohols, Phenols and Ethers:
Alcohols: Classification, general formula, structure and nomenclature. Methods of preparation, physical and chemical properties (of primary alcohols only), identification of primary, secondary and tertiary alcohols, mechanism of dehydration, uses with special reference to methanol and ethanol.
(i) Classification into monohydric, dihydric and polyhydric alcohols, general formula, structure and nomenclature of alcohols. Difference between primary, secondary and tertiary alcohols in terms of structure, physical properties and chemical properties.
(ii) Methods of preparation:
- Hydration of Alkenes – direct hydration, indirect hydration, hydroboration oxidation.
- From Grignard’s reagent.
- Hydrolysis of alkyl halides.
- Reduction of carbonyl compounds.
- From primary amines.
Properties:
- Acidic nature of alcohols
- Reaction with sodium.
- Esterification with mechanism.
- Reaction with hydrogen halides.
- Reaction with PCl3, PCl5, and SOCl2.
- Reaction with acid chlorides and acid anhydrides
- Oxidation
- Dehydration
Uses of alcohols.
(iii) Conversion of one alcohol into another
(iv) Distinction between primary, secondary and tertiary alcohols by Lucas’ Test.
Phenols: Classification and nomenclature. Methods of preparation, physical and chemical properties, acidic nature of phenol, electrophilic substitution reactions, uses of phenols.
Preparation of phenol from diazonium salt, chlorobenzene (Dow’s process) and from benzene sulphonic acid.
Manufacture from Cumene.
Physical properties: state and solubility
Chemical properties:
- Acidic character of phenol.
- Reaction with sodium hydroxide.
- Reaction with sodium.
- Reaction with zinc.
- Reaction with acetyl chloride and acetic anhydride.
- Reaction with phosphorus pentachloride.
- Bromination, nitration and sulfonation (Electrophilic substitution reactions).
- Kolbe’s reaction (formation of salicylic acid).
- Reimer – Tiemann reaction
- Test for phenol – FeCl3test, azo dye test.
Aliphatic Ethers: General formula, structure and nomenclature. Methods of preparation, physical and chemical properties, uses.
Ethers: structure of ethereal group.
Preparation from alcohol (Williamson’s synthesis).
Physical properties: state, miscibility.
Chemical properties:
- Reaction with chlorine.
- Oxidation (peroxide formation).
- Reaction with HI.
- Reaction with PCl5.
Aryl ethers
Physical properties – state and solubility.
Chemical properties – preparation of anisole (Williamson’s synthesis), electrophilic substitution (halogenation, nitration and Friedel-Crafts reaction.) Uses of ether.
12. Aldehydes, Ketones and Carboxylic Acids
Nomenclature, structure of methods of preparation of aldehydes and ketones, physical and chemical properties, mechanism of nucleophilic addition, reactivity of alpha hydrogen in aldehydes and uses.
Preparation:
- From alcohol
- From alkenes (ozonolysis).
- From alkynes (hydration).
- From acid chlorides (Rosenmund reduction, reaction with dialkyl cadmium).
- From calcium salt of carboxylic acids.
- From nitriles (Stephen reaction, Grignard’s reagent).
- From esters.
Physical properties – state and boiling point.
Chemical properties:
- Nucleophilic addition reactions with mechanism (ammonia and its derivatives, HCN, NaHSO3 and Grignard’s reagent).
- Oxidation reactions, iodoform reaction.
- Reduction: reduction to alcohol and alkanes (Clemmensen reduction, Wolff-Kishner reduction, Red phosphorus and HI).
- Base catalysed reactions (with mechanism): Aldol condensation, cross Aldol condensation, Cannizzaro’s reaction.
Tests: difference between formaldehyde and acetaldehyde; aldehydes and ketones.
Uses of aldehydes and ketones.
Aromatic aldehyde (Benzaldehyde)
Lab preparation from toluene by oxidation with chromyl chloride.
Physical properties: state and stability.
Chemical properties:
- Oxidation and reduction.
- Nucleophilic addition reaction (hydrogen cyanide and sodium bisulphite).
- Reactions with ammonia and its derivatives (hydroxylamine, hydrazine and phenylhydrazine).
- Reaction with phosphorus pentachloride.
- Cannizzaro reaction.
- Benzoin condensation.
- Perkin’s reaction.
- Electrophilic substitution – halogenation, nitration and sulphonation.
Test: distinction between aromatic and aliphatic aldehydes.
Uses of benzaldehyde.
Carboxylic Acids:
Classification, general formula and structure of carboxylic group. Nomenclature, acidic nature, methods of preparation, physical and chemical properties and uses.
Classification of mono and di carboxylic acids with examples.
Preparation of aliphatic and aromatic carboxylic acid:
- From alcohols, aldehydes.
- From nitriles.
- From Grignard’s reagent
Physical properties: state, boiling point and solubility.
Chemical properties:
- Acidic character: (aliphatic, aromatic carboxylic acids with the effect of substituents on the acidic character – to be dealt with in detail) –
- Reaction with active metals, alkalies, carbonates and bicarbonates,
- Formation of acid derivatives.
- Decarboxylation (chemical and Kolbe’s electrolytic reaction).
- HVZ reactions.
- Substitution of benzene ring (meta directive effect of carboxylic acid group) nitration and sulphonation.
Tests for acids: formic acid, acetic acid and benzoic acid.
Uses of formic acid, acetic acid and benzoic acid.
13. Organic compounds containing Nitrogen
Aliphatic Amines: General formula and, classification of amines. Structure of the amino group, nomenclature. Methods of preparation, physical and chemical properties, uses, identification of primary, secondary and tertiary amines.
- Amines
Nomenclature, classification with examples, structure, general formula.
Methods of preparation:
- From alcohol.
- From alkyl halide.
- From cyanide.
- From amide (Hofmann degradation).
- From nitro compounds.
- Gabriel phthalimide Synthesis.
Physical properties: comparison between primary, secondary and tertiary amines in terms of – state, solubility, boiling point (hydrogen bonding), comparison with alcohols.
Chemical properties:
- Basic character of amines
- comparison between primary, secondary and tertiary alkyl amines/ ammonia/ aniline. Effect of substituents on the basic strength of aniline
- Alkylation and acylation with mechanism. – Reaction with nitrous acid.
- Carbylamine reaction.
Distinction between primary, secondary and tertiary amines (Hinsberg’s Test).
Aniline
Preparation reduction of nitrobenzene.
Physical properties – state, solubility and boiling point.
Chemical properties:
- Reaction with HCl and H2SO4.
- Acetylation, alkylation.
- Carbylamine reaction.
- Electrophilic substitution (bromination, nitratio
n and sulphonation).
Tests for aniline.
Uses of aniline.
Cyanides and Isocyanides
Methods of preparation:
Cyanides:
- From alkyl halide.
- From amide.
Isocyanides:
- From alkyl halide.
- From primary amines
Diazonium salts:
Preparation, chemical reactions and importance in synthetic organic chemistry. Preparation from aniline;
Properties: Sandmeyer’s reaction, Gattermann reaction and Balz–Schiemann reaction, replacement of diazo group by -H, -OH, -NO2, coupling reaction with phenol and aniline.
14. Biomolecules
Carbohydrates – Definition, Classification (aldoses and ketoses), monosaccharides (glucose and fructose), D-L configuration oligosaccharides (sucrose, lactose, maltose), polysaccharides (starch, cellulose, glycogen); Importance of carbohydrates.
Carbohydrates: definition, classification – mono (aldose, ketose), oligo (di, tri, tetra saccharides) and polysaccharides with examples: reducing sugars and non reducing sugars – examples and uses.
Establishment of structures for glucose and fructose (open and cyclic) heating with HI, reaction with hydroxylamine, bromine water, acetic anhydride, nitric acid and phenyl hydrazine.
Test for glucose and fructose (bromine water test with equation).
Disaccharides – structures of sucrose, maltose and lactose (glycosidic linkage).
Polysaccharides – starch, cellulose, glycogen.
Proteins – structural units of proteins. Basic idea of – amino acids, peptide bond, polypeptides, proteins, structure of proteins – primary, secondary, tertiary structure and quaternary structures (qualitative idea only), denaturation of proteins. Enzymes, hormones – elementary idea only.
Proteins: Amino acids – general structure, classification and zwitter ion formation.
Isoelectric point.
Classification of proteins on the basis of molecular shape; primary, secondary, tertiary and quaternary, structures of proteins, denaturation of proteins. (Definitions only. Details and diagrams are not required).
Vitamins – Classification and functions. Vitamins A, B, C, D, E and K: classification (fat soluble and water soluble), deficiency diseases. (Chemical names and structures are not required).
Nucleic Acids – DNA and RNA. Nucleic acids: basic unit – purine and pyrimidine, DNA – structure (double helical), RNA (No chemical structure required). Differences between DNA and RNA.
15. Polymers
Definition and classification on different parameters. Methods of polymerisation (addition and condensation), copolymerisation, and some important polymers: natural and synthetic like polythene, nylon polyesters, bakelite, rubber. Biodegradable and nonbiodegradable polymers.
Classification based on source, on structure, on mode of polymerisation, on molecular forces, on growth (with free radical mechanism). Preparation of important addition polymers – Polythene, polypropene, PVC, PTFE, polystyrene.
Rubber – natural and synthetic (Buna-N and Buna-S), vulcanisation of rubber.
Preparation of important condensation polymers
polyester, Nylon 66, Nylon 6, Bakelite, melamine (to be learnt in terms of monomers and equations).
Biodegradable polymers – PHBV, Nylon 2 – Nylon 6.
16. Chemistry in Everyday life
Chemicals in medicines – analgesics, tranquilizers antiseptics, disinfectants, antimicrobials, antifertility drugs, antibiotics, antacids, antihistamines.
In medicine: antipyretics, analgesics, tranquilizers, antiseptics, disinfectants, 187 antimicrobials, anti-fertility drugs, antihistamines, antibiotics, antacids.
Definition, common examples, uses.
Differences between antiseptics and disinfectants. Structure not required.
Chemicals in food – preservatives, artificial sweetening agents, elementary idea of antioxidants.
Preservatives: role, example (Sodium benzoate).
Artificial sweetening agents: role, examples (aspartame, saccharin, sucralose and alitame).
Soaps and detergents – Classification and their cleansing action.
Soaps and detergents: classification, structure and some important examples.
Advantage of detergents over soaps; classification of detergents into anionic/biodegradable, cationic/non-biodegradable and non-ionic.
Described Syllabus For Practical Exam:
Candidates are required to complete the following experiments:
- Titrations
Oxidation-reduction titrations: potassium manganate (VII) / ammonium iron (II) sulphate; potassium manganate (VII) / oxalic acid. The candidate may be required to determine the percentage purity of a compound and the number of molecules of water of crystallization in hydrated salts. In such experiments sufficient working details including recognition of the end point will be given.
Candidates will be required to calculate:
- Molarity
- Concentration in grams L-1 / molecular mass
- Number of molecules of water of crystallisation/ percentage purity.
NOTE: Molarity must be calculated upto 4 decimal places at least, in order to avoid error.
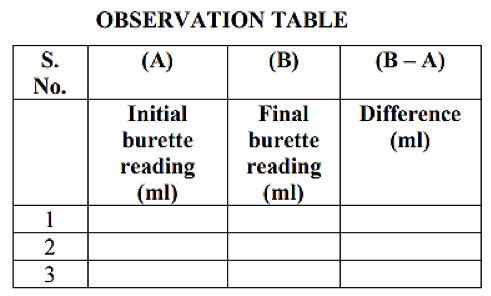
- Concordant reading is to be used for titre value. Concordant reading is two consecutive values which are exactly the same. Average will not be accepted as titre value.
- The table is to be completed in ink only. Pencil is not to be used.
- Overwriting will not be accepted in the tabular column.
Observations:
- Pipette size (should be same for all the candidates at the centre).
- Titre value (concordant value).
- Study of the rate of reaction
The candidates will be required, having been given full instructions, to carry out an experiment on the rate of reaction, e.g. reaction between sodium thiosulphate and hydrochloric acid (using different concentrations for either), magnesium and dil. sulphuric acid/ dil. hydrochloric acid (using different concentrations).
- Graph of volume vs. time and its interpretation.
- Relationship between concentration and rate, volume and rate and time and rate.
- Identification of the following compounds and functional groups based on observations
Alcoholic group – glycerol
- Aldehyde group- formaldehyde
- Ketonic group – acetone
- Carboxylic group – benzoic acid
- Amino group – aniline
Please Note: Carbylamine and acrolein tests should not be performed.
The student should learn to differentiate between colours, solution, ring and precipitate.
- Characteristic tests of carbohydrates and proteins
- Carbohydrates – glucose
- Proteins – powdered milk
Identification should be of ‘Carbohydrate’ and ‘Protein’ not of individual substances.
- Experiments related to pH change using pH paper or universal indicator.
- Determination of pH of some solutions obtained from fruit juice, solutions of known and varied concentrations of acids, bases and salts.
- Comparison of pH of the solutions of strong and weak acids of the same concentration.
Use of universal indicator/pH paper must be taught to the students.
- Electrochemistry
Setting up a simple voltaic cell.
Variation of cell potential in Zn/Zn2+//Cu2+/Cu with change in concentration of electrolyte (CuSO4, ZnSO4) at room temperature.
- Qualitative analysis
Qualitative analysis: identification of single salt containing one anion and one cation:
- Anions: CO32-, NO2 – , S2-, SO32-, SO42-, NO3 – , CH3COO– , Cl– , Br– , I– , C2O42-, PO43-.
- Cations: NH4+, Pb2+, Cu2+, Al3+, Fe3+, Zn2+, Mn2+ Ni2+, Co2+, Ba2+, Sr2+, Ca2+, Mg2+.
NOTE: Chromyl chloride test not to be performed. For wet test of anions, sodium carbonate extract must be used (except for carbonate). (Insoluble salts such as lead sulphate, barium sulphate, calcium sulphate, strontium sulphate will not be given).
Anions:
- Dilute acid group – CO32-, NO2 – , S2-, SO32-
- Concentrated Acid Group – NO3 – , CH3COO– , Cl– , Br– , I–.
- Special Group – SO42-, PO43-, C2O42-.
Cations:
- Group Zero: NH4+
- Group I: Pb2+
- Group II : Cu2+, Pb2+
- Group III: Al3+, Fe3+
- Group IV: Zn2+, Mn2+, Ni2+, Co2+
- Group V: Ba2+, Sr2+, Ca2+
- Group VI: Mg2+
NOTE:
- Formal analytical procedure is required for Qualitative Analysis.
- Specific solvent for O.S. to be used;
- Before adding Group III reagents to the filtrate of Group II, H2S must be removed followed by boiling with conc. Nitric acid.
- The right order for buffer (NH4Cl and NH4OH) must be used.
- The flame test with the precipitate obtained in Group V for Ba2+, Sr2+, Ca2+ will also be accepted as a confirmatory test.
For wet test of anions, sodium carbonate extract must be used (except for carbonate).
Suggested Books:
- Nootan ISC Chemistryfor Class 12 by HC Srivastava
- S Chand ISC Chemistryfor Class 12 by RD Madan and BS Bisht
- Physical and Inorganic Chemistry by KL Chugh


